Abstract
Background:
CAR T-cell therapy (CAR T) has become a powerful new tool in the treatment of relapsed/refractory (r/r) hematologic malignancies. Infections are well-recognized risks of CAR T. Bridging therapy is often employed to allow patients to reach successful infusion but may also further immunosuppress this already vulnerable group. This study aims to identify patterns of infections post-CAR T and the potential impact of bridging therapy.
Methods:
Medical records of patients who underwent apheresis for CAR T from 2018-2022 for r/r lymphomas or multiple myeloma (MM) at UT Southwestern were analyzed. Demographics, treatment characteristics and infection data were collected via retrospective chart review. Post-CAR T infections were identified by reviewing patient hospitalizations after CAR T infusion for infectious complications confirmed by positive diagnostic studies during admission. Infections were categorized as bacterial, viral, fungal, or multi-pathogenic. Time-to-infection was calculated from lymphodepletion (LD) date. Categorical variables were compared using Fisher's exact test. Continuous variables were compared using Mann-Whitney U test. Overall- and progression-free survival (OS and PFS) were calculated from CAR T infusion date, estimated by the Kaplan-Meier method, and compared using a log-rank test.
Results:
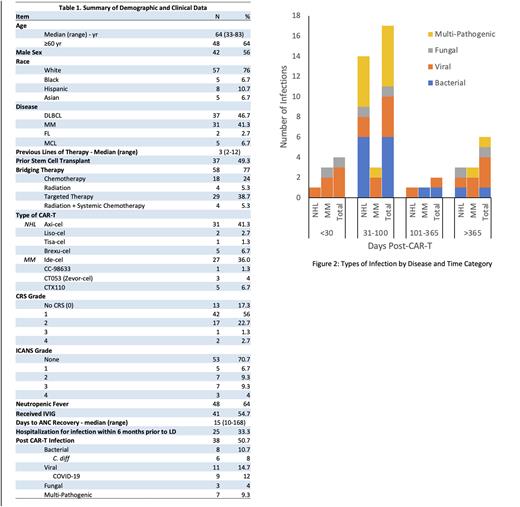
Data were collected on 75 total patients, 44 with lymphoma and 31 with MM, who underwent apheresis and 74 (98.7%) underwent CAR T. Baseline patient characteristics are shown in Table 1. All patients received standardized opportunistic infection (OI) prophylaxis and intravenous immunoglobulin (IVIG) and growth factor support based on institutional guidelines.
Thirty-eight patients (51%) had a post-CAR T infection. Post-CAR T infections manifested at a median of 117 days (range: 12 - 1344 days) after LD. Patients who received bridging therapy had an earlier median incidence of post CAR T infections (92 vs 113 days post-LD, p = 0.03). Number of prior lines of therapy did not predict infection rates or time to infection. Time-to-infection did not vary by lymphoma versus MM or by cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity (ICANS) grade. MM patients had a numerically higher rate of viral infections (77% vs 41%) while lymphoma patients had a higher bacterial infection rate (65% vs 55%). A majority (58%) of these patients had infectious events between 31- and 100-days post lymphodepletion [Figure 2]. Infection rates were not significantly associated with tocilizumab or steroid usage during CAR T.
In our cohort, 77% of patients received bridging. Bridging therapy was associated with a significantly higher neutropenic fever rate (73% vs 37%, p = 0.006), which persisted after adjusting for prior lines of therapy. All patients with neutropenic fever received broad-spectrum antibiotic coverage. In lymphoma patients, bridging therapy was associated with higher grades of CRS (p = 0.018). Being hospitalized for infection within 6 months (p=0.01) or receiving antibiotics in the month (p=0.02) prior to lymphodepletion, were associated with grade ≥3 CRS.
One-year PFS and OS were 55% and 74% for the entire cohort. Four patients died due to infectious complications after successful CAR T infusion at a median of 163 days post infusion. Patients who had post-CAR T infections trended towards worse progression-free survival (1-year PFS 65% vs 46%, p = 0.1) and had similar OS (1-year OS 70% vs 77%, p = 0.75).
Conclusions:
Bridging therapy prior to CAR T may increase the risk of neutropenic fever and be associated with earlier infectious risk. Febrile neutropenia and high-grade CRS drive more intensive resource utilization, which may be mitigated with more intentional patient selection for bridging therapy.Our findings show that post-CAR T infections contribute to significant morbidity, and thus calls for personalizing bridging strategies, peri-CAR T OI prophylaxis and infection management.
Disclosures
Anderson:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kaur:UT Southwestern: Consultancy, Current Employment. Madanat:Sierra Oncology, Stemline Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees; BluePrint Medicines, GERON, OncLiv: Consultancy, Honoraria. Awan:Pharmacyclics: Consultancy, Research Funding; Gilead Sciences: Consultancy; Kite Pharma: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; Janssen: Consultancy; AbbVie: Consultancy; Johnson and Johnson: Consultancy; BeiGene: Consultancy; Dava Oncology: Consultancy; BMS: Consultancy; Incyte: Consultancy; Verastem: Consultancy; MEI Pharma: Consultancy; Cardinal Health: Consultancy; Merck: Consultancy; ADCT Therapeutics: Consultancy; Cellecter Bisosciences: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Caribou Biosciences: Consultancy. Ramakrishnan Geethakumari:Kite: Consultancy; BMS: Consultancy; Rafael Pharma: Consultancy; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cellectar Biosciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.